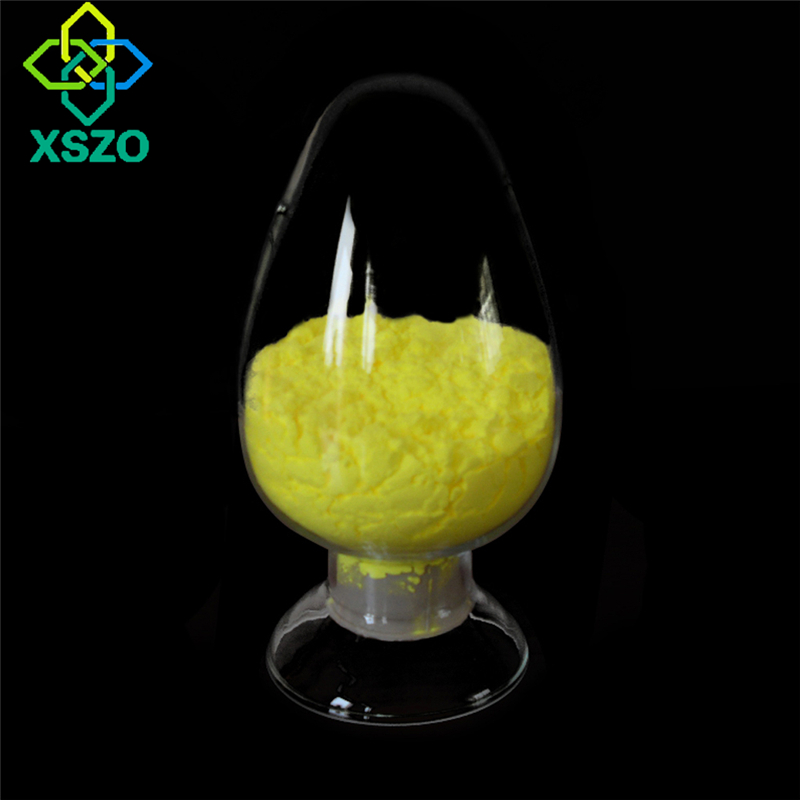
Application of sulfobutyl beta cyclodextrin sodium in insoluble drugs
2022/01/11
Cyclodextrin is mainly used to increase the solubility of poorly soluble drugs, improve drug stability, and reduce adverse reactions and irritation. The solubilization mechanism includes the functions of inclusion, non-inclusion, aggregation and surfactant, and promoting the supersaturation and stable supersaturation of the dissolved drug. Venuti et al. used UV-Raman spectroscopy and Fourier transform attenuated total reflection infrared spectroscopy to record the hydrogen bond vibration spectra between water molecules and between water molecules and SBE-B-CD, and simulated them with generalized least squares and curve functions. The method was analyzed. It was observed that as the concentration of cyclodextrin in the aqueous solution increased, the average hydrogen bond strength and average hydrogen bond enthalpy decreased. The molecular explanation of the solubilization mechanism of sulfobutyl beta cyclodextrin sodium is to destroy the maintenance solvent Hydrogen bonding in which water molecules are arranged in an orderly tetrahedron
Contract cooperation
Inclusion means that the hydrophobic group of the poorly soluble drug enters the hydrophobic cavity of the cyclodextrin under the matching of the three-dimensional structure and the polarities of the two. The host molecule and guest molecule are mainly combined by non-covalent bonding, such as dispersion force, gravitational force between dipoles, hydrogen bond, charge mobility, etc., generally a synergistic effect of several forces, which manifests as a physical dynamic Balancing process. In dilute solutions, inclusion is the main form, or even the only form, of the combination of drugs and cyclodextrins. It is worth noting that sulfobutyl beta cyclodextrin sodium is different from other cyclodextrin derivatives. The charged sulfonic acid group is far away from the cyclodextrin matrix and will not fold into the cavity of cyclodextrin. Affect hydrophobicity. Moreover, because the charges of the sulfonic acid groups repel each other away, a hydrophobic region composed of the alkyl ether portion of sulfobutyl is formed near the cyclodextrin cavity, which jointly participates in the inclusion of drug molecules.