Introduction of NMN (β-nicotinamide mononucleotide) and its relationship and difference with other NAD+ precursors
2022/01/11
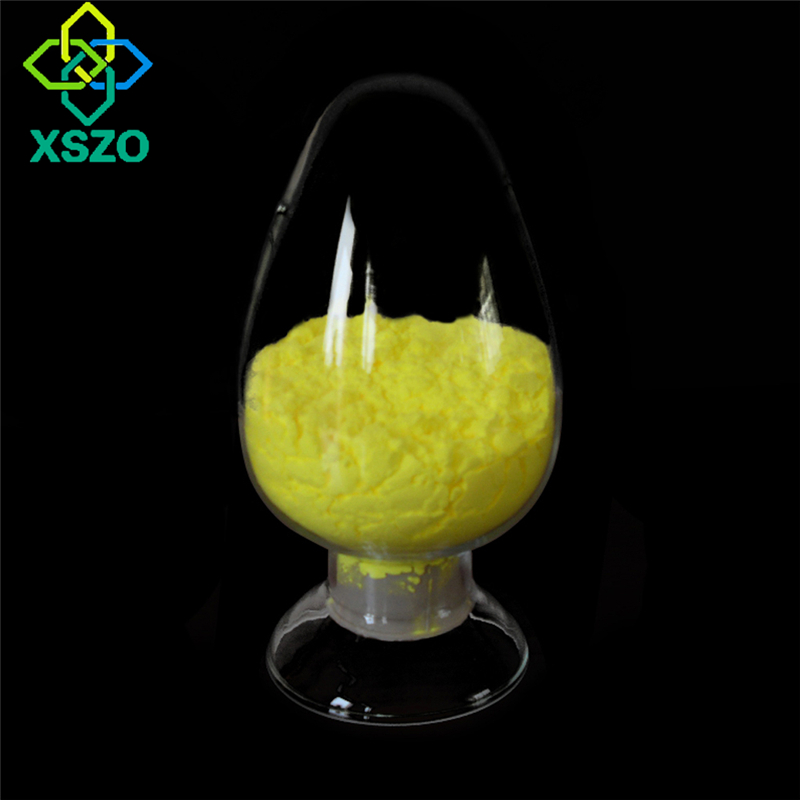
Nicotinamide mononucleotide (NMN) is a derivative of vitamin B3 (nicotinamide) with a molecular weight of 334.221g/mol[1]. There are two epimers of NMN: α type and β type. Studies have found that only β-type NMN has biological activity [2].
NMN naturally exists in various foods. For example, every 100g of cauliflower and cabbage contains 0.25-1.12mg and 0-0.9mg NMN; every 100g avocado and tomato contain 0.36-1.6mg and 0.26-0.3mg NMN, and each 100g raw beef only contains 0.06-0.42mg NMN[3].
The main role of NMN is to act as an intermediate in the biosynthesis of NAD+ in the human body. In the process of biosynthesis of NAD+, NMN is a nicotinamide mononucleotide adenosyl transferase 1 (NMNAT1) derived from the nucleus and nicotinamide mononuclear derived from mitochondria. An important substrate of NMNAT3 (NMNAT3) [4]. Studies have found that NAD+ levels steadily decay with age, leading to changes in metabolism and increased disease susceptibility; restoring NAD+ levels in elderly or diseased animals can promote health and extend lifespan [5].
Preclinical studies have shown that NMN has a variety of pharmacological effects in heart and cerebral ischemia, Alzheimer`s disease, diet and age-induced type 2 diabetes and obesity, which are all related to the lack of NAD+[6] [7] [8].
Most clinical trials of oral NMN metabolism are still in progress[9]. At present, a publicly published human clinical trial of NMN has proved that a single oral administration of 100, 250, 500 mg of NMN will not cause side effects such as blushing and gastrointestinal reactions. And NMN will be efficiently metabolized by the human body without causing any obvious harmful effects [10].
2 Relationship and difference with other NAD+ precursors
NMN is structurally similar to other NAD+ precursors such as nicotinamide ribose (NR), niacin (NA) and nicotinamide (NAM), and both contain a pyridine ring structure; but niacin (NA) and nicotinamide (NAM) are There are some shortcomings in application, such as nicotinamide can cause nausea and blushing, and it is reported that taking nicotinamide in high doses can cause liver toxicity [11]. A new preclinical study shows that nicotinamide (NAM) stays in rats longer than NMN
Short [12]. Niacin (NA) as an immediate-release preparation can cause skin flushing and other adverse reactions when used, and as a sustained-release preparation, it may cause liver toxicity [13]. Among the NAD+ precursors, nicotinamide ribose (NR) and NMN are exceptions. The adverse side effects of these two metabolites are rarely reported [14]. NR and NMN have the same oral bioavailability.