The difference between adenosine triphosphate disodium and adenosine triphosphate
2022/01/11
Adenosine disodium triphosphate is a nucleotide derivative that participates in the metabolism of fat, protein, sugar, nucleic acid and nucleotides in the body. When energy is needed for absorption, secretion, muscle contraction and biochemical synthesis in the body, adenosine triphosphate is decomposed into diphosphate Adenosine and phosphate groups simultaneously release energy.
Adenosine disodium triphosphate is a nucleotide derivative that participates in the metabolism of fat, protein, sugar, nucleic acid and nucleotides in the body. When energy is needed for absorption, secretion, muscle contraction and biochemical synthesis in the body, adenosine triphosphate is decomposed into diphosphate Adenosine and phosphate groups simultaneously release energy.
Adenosine disodium triphosphate can improve the stability and reconstruction ability of nerve cell membrane structure, and promote the re-growth of neurites. This product and pentose can synthesize nucleic acid under the action of enzymes in the body, and the effect of phospholipid cholamine in transcytidase It can synthesize cephalin and cytidine monophosphate.
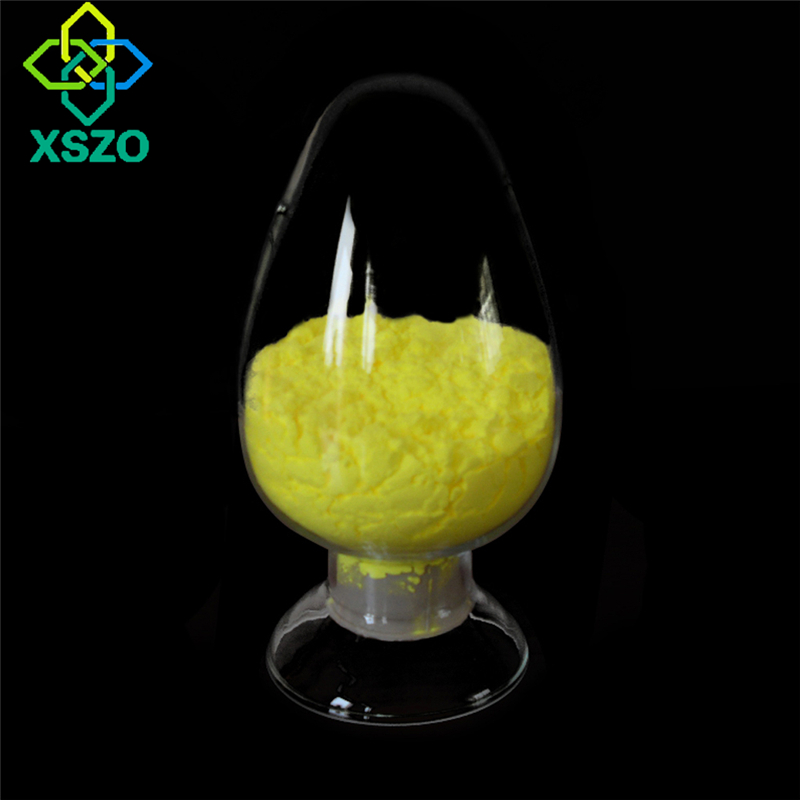
Adenosine triphosphate is a high-energy compound made with Xanthine nucleotide as a substrate through biological fermentation technology. Adenosine triphosphate is the direct source of energy required for all life activities of tissue cells in the body, and is known as the "molecular currency" of intracellular energy. Storage and transmission of chemical energy, the synthesis of proteins, fats, sugars and nucleotides all require its participation, which can promote the repair and regeneration of various cells in the body, enhance the metabolic activity of cells, and have a strong target for the treatment of various diseases .
ATP structure:
chemical properties
ATP is composed of adenosine and three phosphate groups, the molecular formula is C10H16N5O13P3. The chemical formula is C10H8N4O2NH2(OH)2(PO3H)3H, and the molecular weight is 507.184. The three phosphate groups are categorized into α, β and γ phosphate groups starting from adenosine. , The chemical name of ATP is 5'-triphosphate-9-β-D-ribofuranosyl adenine, or 5'-triphosphate-9-β-D-ribofuranosyl-6-aminopurine.
Energy supply method
In the conversion between ATP and ADP, the second high-energy phosphate bond of ATP is located at the end, which can be quickly hydrolyzed and broken to release energy. Similarly, it is easy to add the second high-energy phosphate bond under the condition of providing the amount of mutual conversion between ATP and ADP. The 3 phosphoric acid converts ADP into ATP again. For animals and humans, the energy required to convert ADP to ATP comes from respiration. For green plants, the energy required to convert ADP to ATP comes from respiration and photosynthesis, which constitute living cells of organisms. , The internal conversion between ATP and ADP is always carried out, and at the same time it is accompanied by the release and storage of energy.